 Catalysts and batteries
Catalysts and batteries
Elements Strategy Initiative for Catalysts and Batteries (ESICB)
Element Strategy Initiative: To Form Core Research Centers, MEXT
- Observation

-
- Experiment

-

- Developed organic electrolytes with a fire-extinguishing function that are nonvolatile and nonflammable even at 200°C
- Confirmed the potential for stable long-term charging/discharging without use of flammable solvents
- Showed the potential for realizing safe secondary batteries that are larger with higher energy density
Fires and explosions in batteries caused by flammable organic electrolytes
Numerous and varied studies are being conducted on secondary batteries (rechargeable batteries) to produce batteries with higher energy density for use in smartphones and personal computers. Yet there have been multiple incidents of fires and explosions occurring in lithium-ion batteries due to their flammable organic electrolytes. Various types of electrolytes and electrolyte solutions possessing flame-retardant properties have been studied to date, but none have proven practical. Efforts to add flame-retardant solvents to existing electrolytes significantly reduce the battery’s cycle number.
Developing highly concentrated electrolytes with a fire-extinguishing function
At ESICB, we have been conducting research on highly concentrated electrolytes. Concentration of an electrolyte has been determined based on its ionic conductivity. Ordinarily a concentration of 1 mol/L is preferred, but we looked at the unique structures and properties possessed by superconcentrated electrolytes with a > 3 mol/L concentration. Using this concentration, we developed a high-performance organic electrolyte solution with a fire-extinguishing function. The specific composition of this electrolyte includes only flame-retardant trimethyl phosphate (TMP) and a salt (LiN(SO2F)2(LiFSA) or NaN(SO2F)2(NaFSA)). The electrolyte not only has a fire-extinguishing function, but has no flash point and does not become volatile at least up to about 200°C (Figure 1). Moreover, vapor generated when the electrolyte is heated to 200°C or higher acts as a fire-extinguishing agent, proactively mitigating the risk of fire igniting in the battery.
Despite not using any carbonate solvents (flammable), which have been indispensable in the past, we confirmed that this electrolyte allows for a stable charge cycle over a period far exceeding known results with conventional electrolytes and electrolyte solutions for both graphite anodes used in Li-ion batteries and hard-carbon anodes used in Na-ion batteries. An analysis of the results revealed the formation of a robust passivation film derived from anions that is believed to enable a stable charge-discharge cycle.
The fire-extinguishing organic electrolyte discovered in this study solves the current problem of how to produce secondary batteries that are larger and higher in energy density while ensuring high safety.
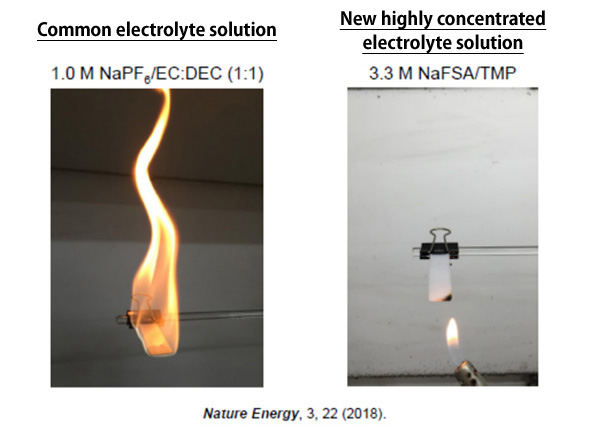
Figure 1 In flame tests to compare flammability, the common electrolyte caught fire when brought near the flame, while the highly concentrated electrolyte not only did not ignite, but actually extinguished the flame
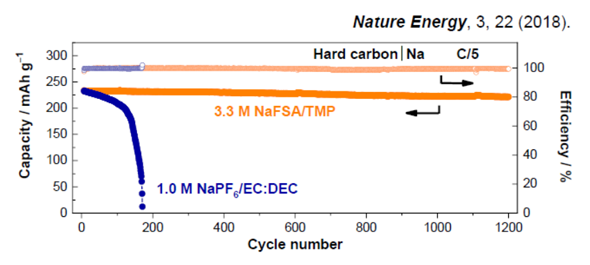
Figure 2 The charge cycle of a hard-carbon anode for an Na-ion battery; the organic electrolyte developed in this study (3.3M NaFSA/TMP) achieved stable charging/discharging for more than 1,200 cycles (over a year and a half)

Atsuo Yamada
School of Engineering
The University of Tokyo
Research collaborator:
Yuki Yamada (School of Engineering, the University of Tokyo)
References:
- [1] Y. Yamada et al., and A. Yamada; J. Am. Chem. Soc., 136, 5039 (2014).
- [2] Y. Yamada et al., and A. Yamada; Nat. Energy, 1, 16129 (2016).
- [3] J. Wang, Y. Yamada et al. and A. Yamada; Nat. Energy, 3, 22-29 (2018).